
Author: C.E. Goo
Publishers: Smashwords, May 22, 2021
Rights and permissions: Open Access. To support scientific research purposes, the internal content of this book is licensed under Creative Commons 4.0, which permits use and sharing, as long as you give appropriate credit to the original author(s) and the source. This book remains the copyrighted property of the author, if you enjoyed this book, please encourage your friends to download their own copy from their favorite authorized retailer, which help to support the ecosystem of author and retailers. Thank you for your support.
ISBN: 9781005536381

Foreword

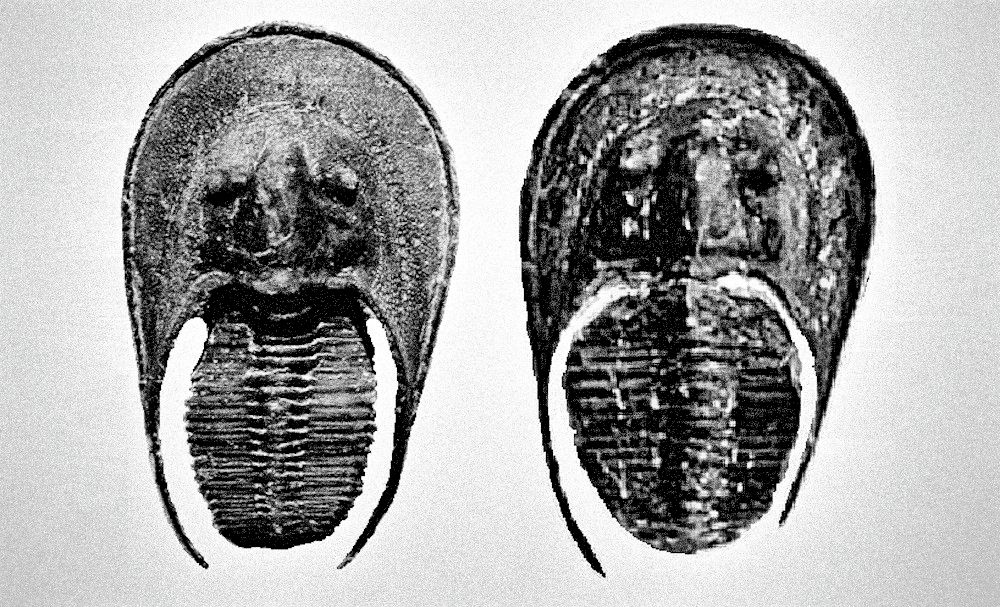
Thank you for your interest in this book. I’m pretty sure you will gain much of knowledge or at least skill to differentiate the male and female of most trilobites after reading this book. You will also notice some language issue for sure but just focus on core concepts within this book that I wish to present to you as my native language is not English. The main point of this book is to discuss how to identify the male and female of trilobites, various types of polymorphism intermediate male and female structure and some common structural behavior of male and female (or possible pregnant posture). This book combines and relates my years of experience in breeding fish and shrimp (at least 15 years) to ancient trilobites to study the possible structural behavior of this extinct arthropod. Below is Crotalocephalus from Morocco that inspired me the trilobites did exhibit the sexual dimorphism among the species and later further discover the existent of polymorphism within a species which will show you some example of female-like male morphs and male-like female morphs at end of the chapter. To commemorate these two specimens, I use them as the cover of this book, and last I hope you will enjoy the interesting topic of this book that I bring to you! Thank you very much and enjoy!

Abstract
Sexual dimorphism refers to exhibition of appearance difference between male and female of the same species especially their size, shape, color or sexual reproductive organs. Study of sexual dimorphism on extinct animal even more complicated as reproductive organ usually does not preserve in fossil specimen. Most of the existing animal and arthropod appear strong sexual dimorphism characteristic among species especially the body shape, using this phenomenon compare to extinct related species will help in study of sexual dimorphism and understand how the evolution of shape difference between male and female and answer some long existing question, did sexual dimorphism is slow accumulate evolution in organism or already occurs in earlier stage once consist of male and female. Trilobites are one of the earliest-known extinct marine arthropods, with preserve abundance fossil specimens and have a very earlier fossil record at about 521 million years ago in the early Cambrian period. Study shows Trilobites did exhibit the sexual dimorphism among their species like existing arthropods, with the general structure of male and female show on Figure 3. Under similar height of similar species, male appear viper head, while female slightly spherical shape; Male contain identical length of both antenna while the female is unequally in length; The body of male appear Hercules muscular shape or looks elongated, the first 6 segments of male thorax tend to have a straight shape of pleural while female appears slightly spherical; The thorax area after the first 6 segments of male typically appear “V” shape and elongated until the end of the pygidium, female usually appear “U” shape structure with board pygidium. Polymorphism is also observed in trilobites, small amount of intermediate-looking individuals actually belong to male-like female morphs (female growth as a male-like pattern) and female-like male morphs (male growth as a female-like pattern). The observation concludes that the first appearance of sexual dimorphism already shows an obvious distinct shape on male and female on Cambrian period.
Introduction
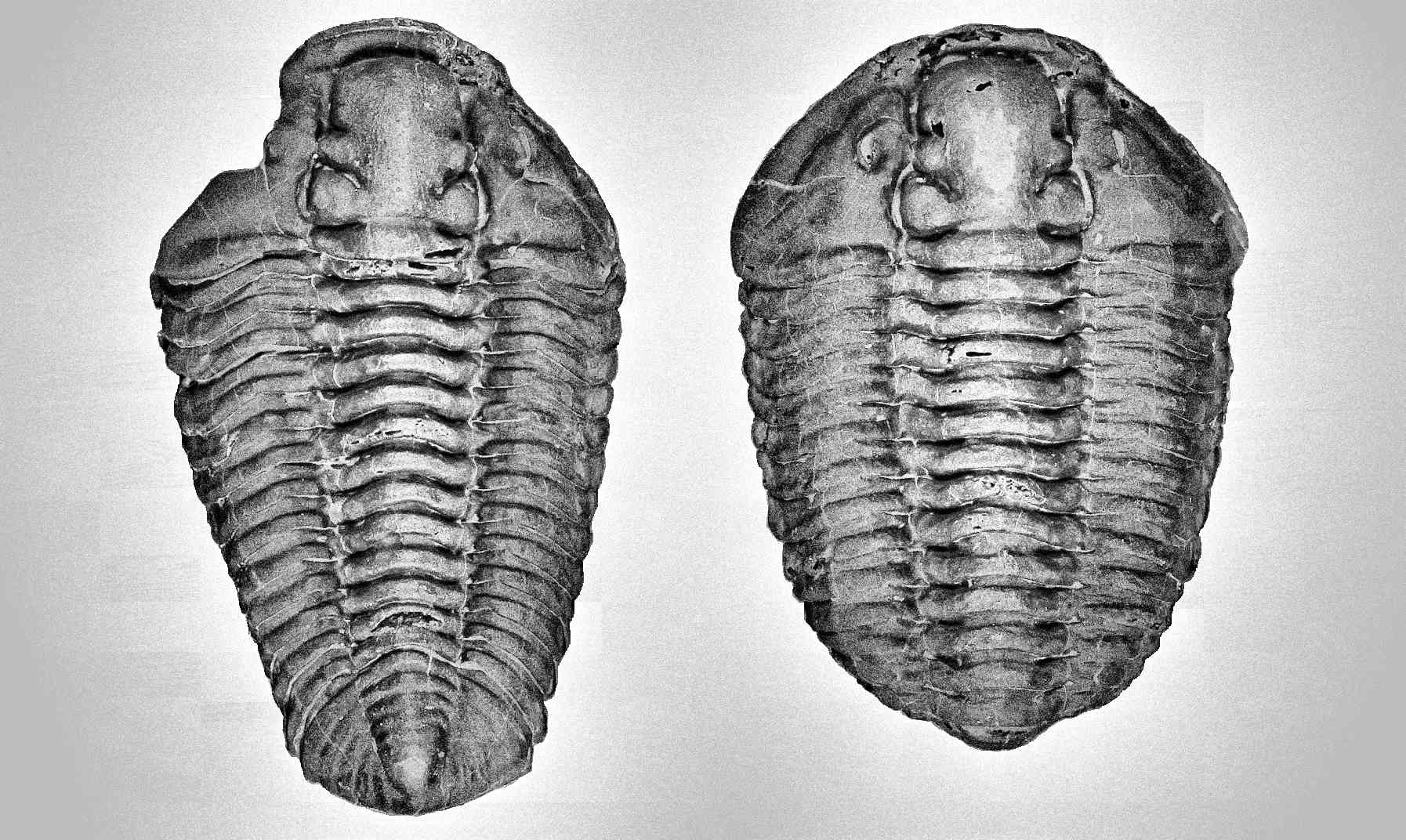
Trilobites, a well known extinct marine arthropod that long being discovered by human via preserved abundant of fossil specimens, but the complexity of morphology and variety of close related species makes identification of male and female trilobites become one of the problematic case studies. Some distinctive variances are often mislabeling as new species or commonly treat as an unremarkable normal individual variance. Since fossils usually do not preserve reproductive organs, this makes it even more difficult in study the differentiation of male and female. Phacops is one of the examples. Phacops alone is huge families of trilobite’s species, typically taxonomy study often grouping trilobite’s species follow the shape of cephalon (head) and pygidium (tail). In fact, the male and female of Phacops did show some distinct morphology shape (refer Figure 26), this characteristic is only notified when more and more Phacops multiple individuals specimen in the same plate has been found and well identify its species. The second thing is trilobites often being referred as one of the close relative to existing horseshoes crab, but actually trilobites have much similarity to exiting marine arthropod, shrimp, especially their behavior. This behavior often exhibits on female species and easily preserved its behavior structure in fossil, and it is one of the useful technique can be used to identify the female morphs (refer Figure 61 & 62). By relating some of the morphology and behavior of existing animals to trilobite preservation structure, it will help us understand more on the probable structure behavior and polymorphism of this extinct marine arthropod.
The study conclusion and evidence show trilobites did exhibit the sexual dimorphism like existing animals, this is done by comparison of huge amount of trilobite’s specimens. The discovery of polymorphism of trilobites helps us understand when did the animal start exhibit appearance different between male and female, plus the earliest knows trilobite appear at about Cambrian period, this indirectly provides us an evidence that the earliest male and female did start show appearance different at about 521 million years ago, the earliest know sexual dimorphism of animal from current study, is long before our predicted and current understanding.
I expect the endocrine system especially sex hormone different between male and female are the core reason that causes sexual polymorphism development among animal, the hormone different affect the growth of animal structure that exhibit the differences. The sexual dimorphism is expected occurs when the first appear of male and female as a reproductive strategy of species continuation of organism.
Methods
Trilobites consist of variant distinct body (thorax) shape, which consist of oval, spherical, rectangle, inverted trapezoid and inverted triangle. Depends on species, some male appear distinctive elongated body shape compare to female in similar age and other appear almost similar in size. The various morphological complexity made difficultly in direct comparison, it required a systematic comparison method to distinguish between male and female especially trilobite that appear rectangle shape, the variance of male and female are very small and difficult to identify.
Under standard comparison method, choosing 2 or more almost same size of trilobite sample, capture a photo in dorsal view and scale to the same height as same as Figure 23. Under digital comparison, most of the distinct shape between male and female will show obviously follow general structure (Figure 3) of Trilobite Guideline.
Discussion
Sexual dimorphisms are commonly found in existing animal. They can be used as a guideline to begin the study of sexual appearance difference in trilobites. Figure.1 shows a main structural difference between male and female of Tri-spined horseshoe crab. The shapes of the prosoma (fore-carapace) are quite different between male and female, and this male character is specific in this species. Males have 6 pairs of marginal spines at both side of back carapace, while female only have 3 pairs of them. This is because males mount here during breeding. The morphology of juveniles is similar in females, but with 6 pairs of marginal spines. Trilobite are slightly different with horseshoe crab, as their male do not mount to female during breeding, they have a structural behavior more similar like existing shrimp. Figure 2 help you understanding the main difference between male and females of existing shrimp. Under this study, we only focus on structural appearance on exoskeleton instead of reproductive organ for comparison purpose as reproductive organ normally do not preserve in trilobites fossil.
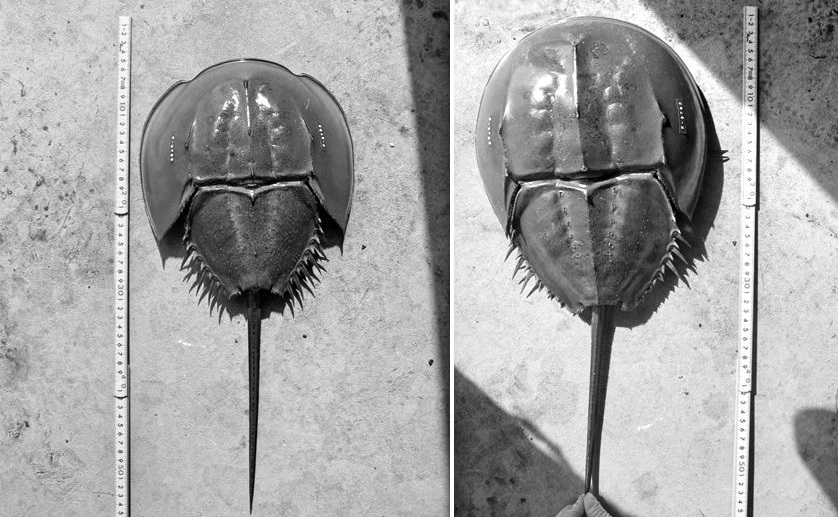

Figure 3 show a general structure guideline for identification of male and female trilobites. Trilobites consist of various distinct shape but they can be simplified into general structure like figure shown below (Figure 3). Note that the unequal length of antenna female trilobites is based on study observation of single species call Triarthrus eatoni (Figure 4) due to its abundance fossils record that preserve soft part details compare to other trilobites species. There are few unique differentiation rules to be remember in order to identify various shape of polymorphs including its basal male and female, the simplified rule are indicate in description of Figure 3.











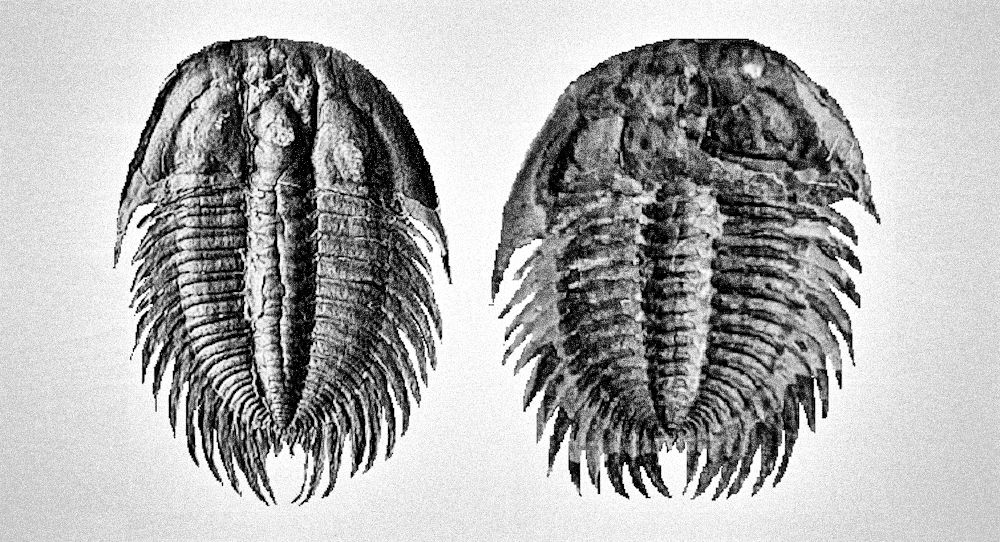








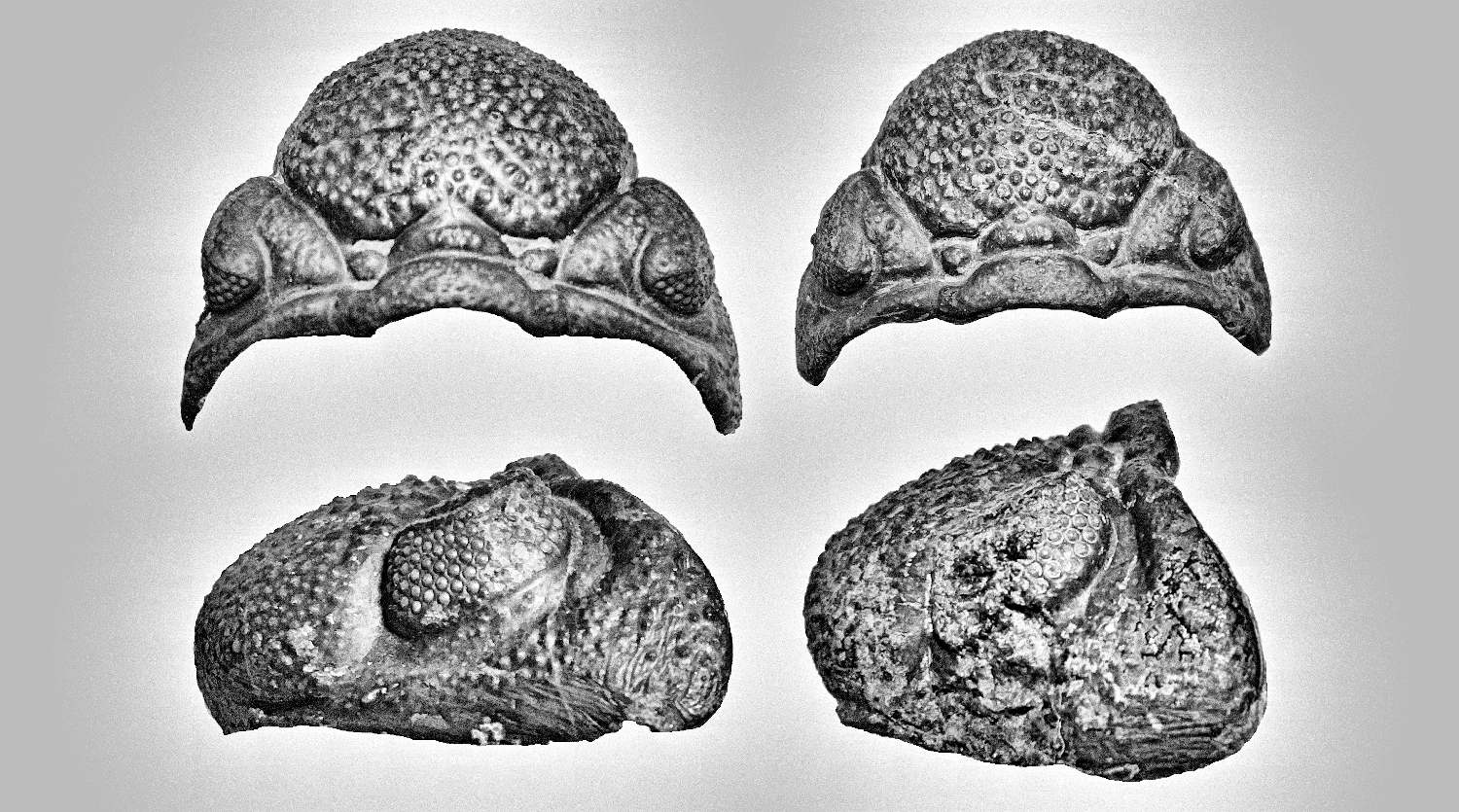







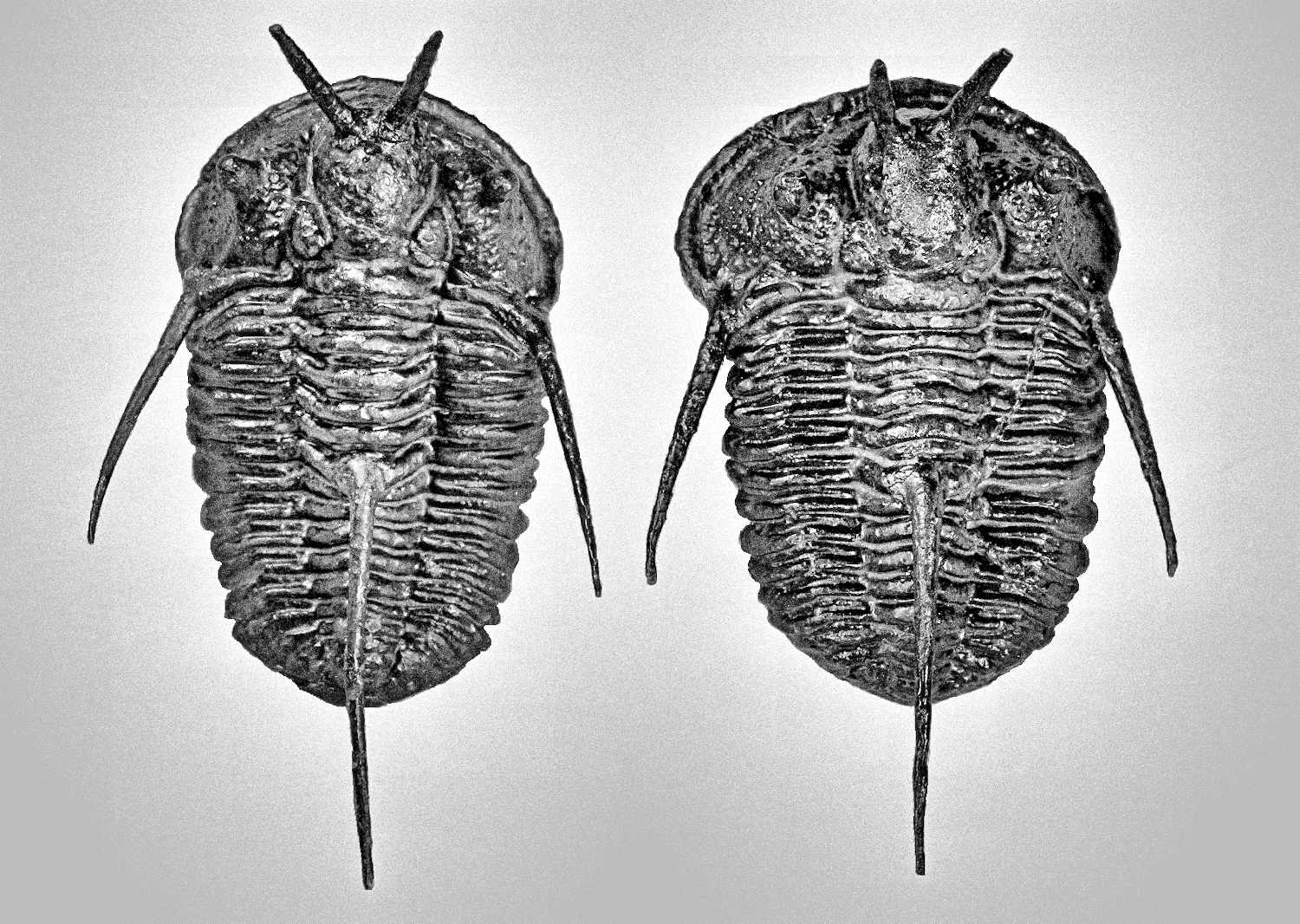
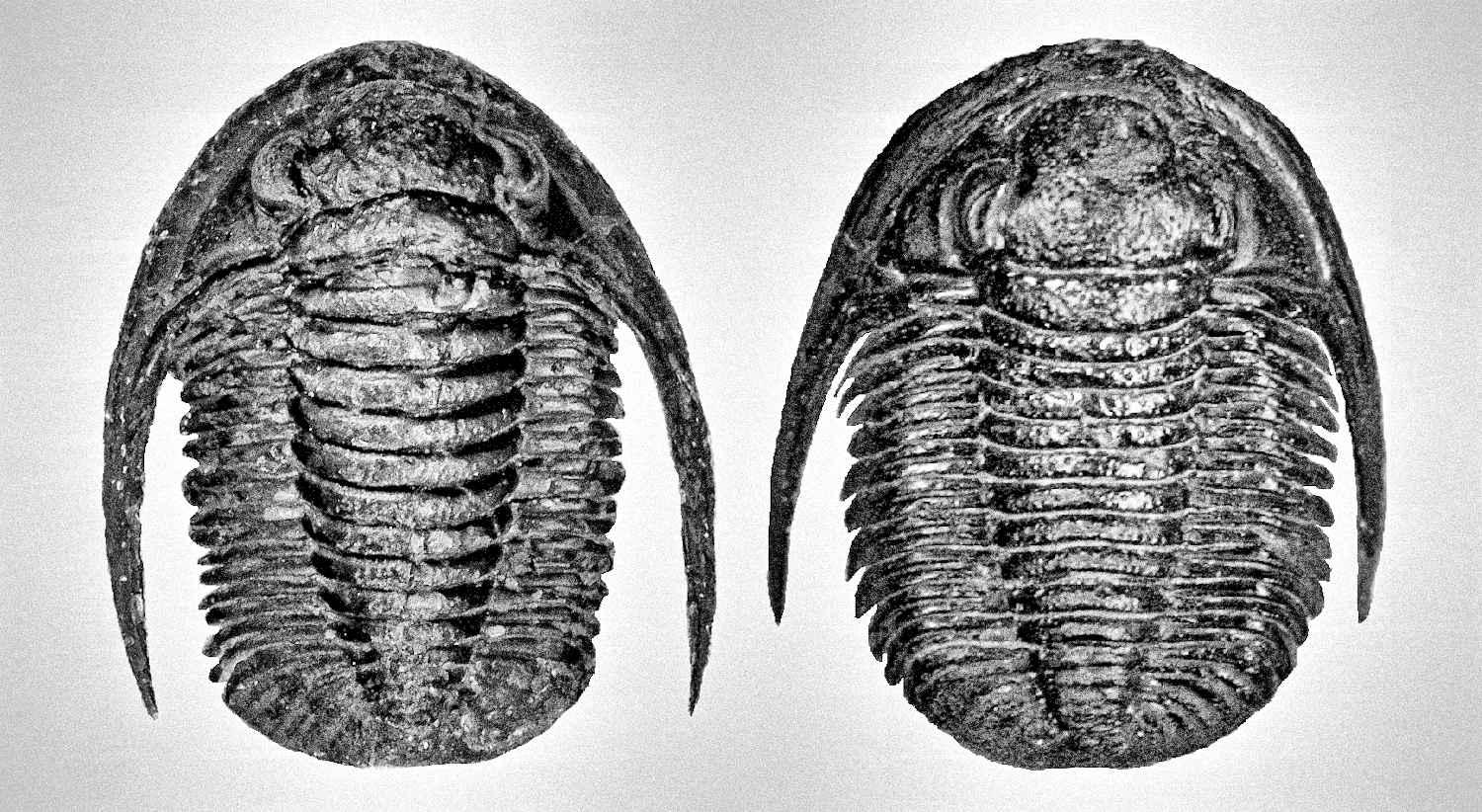

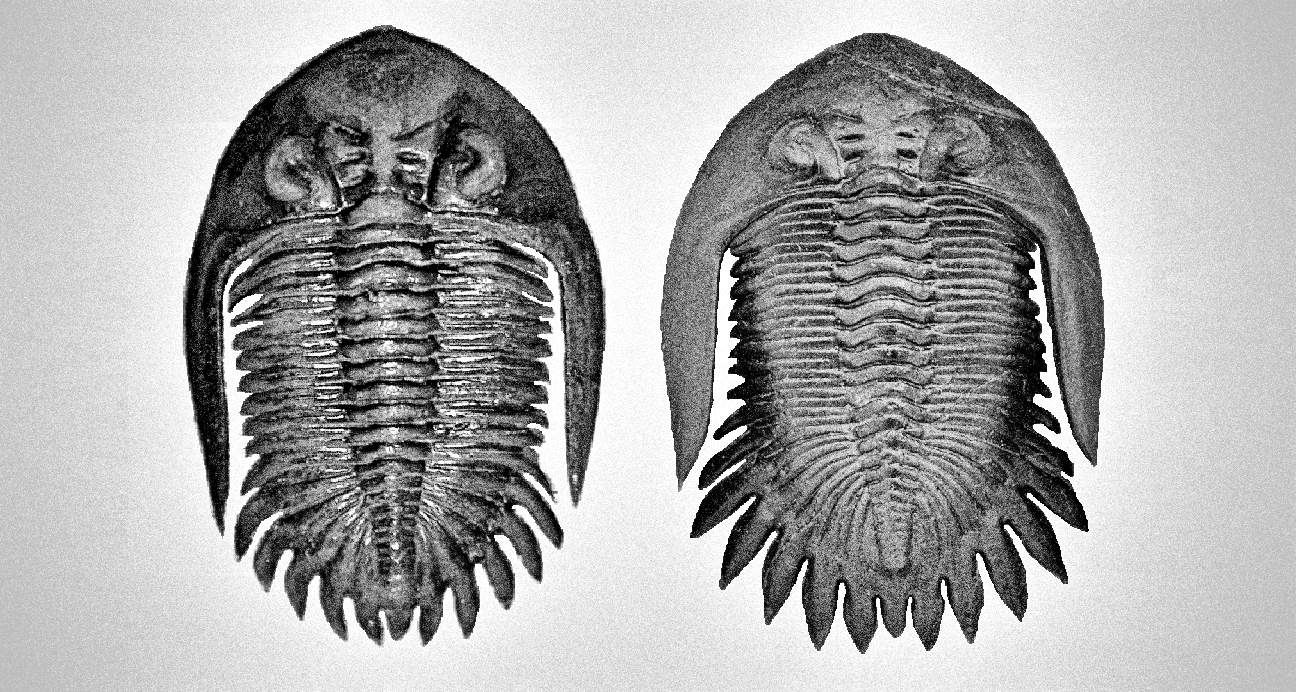

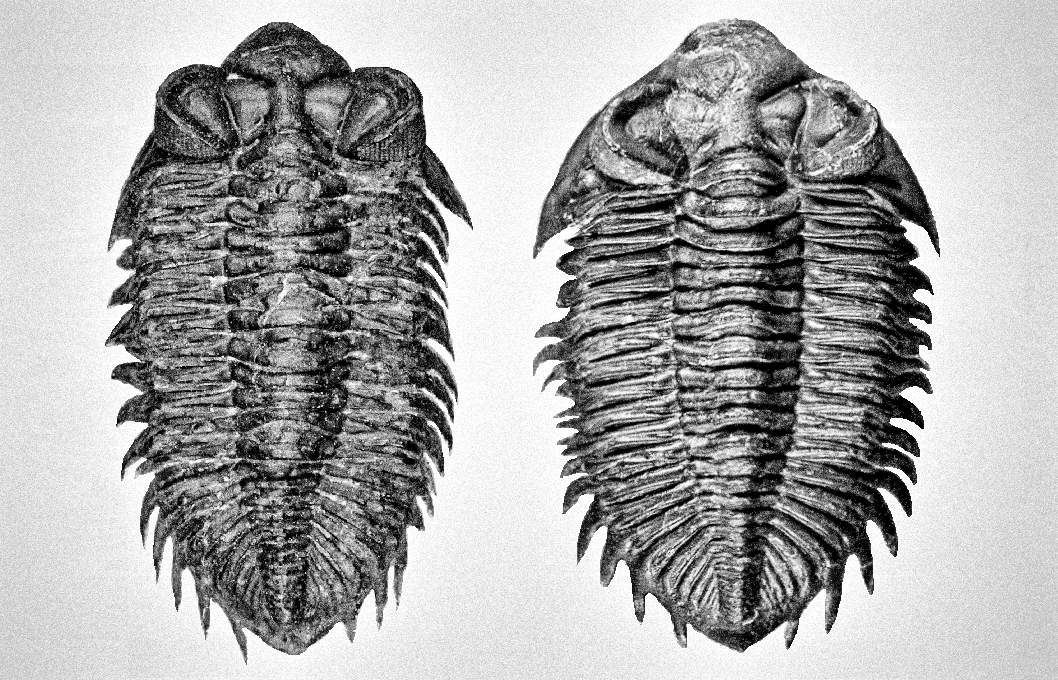
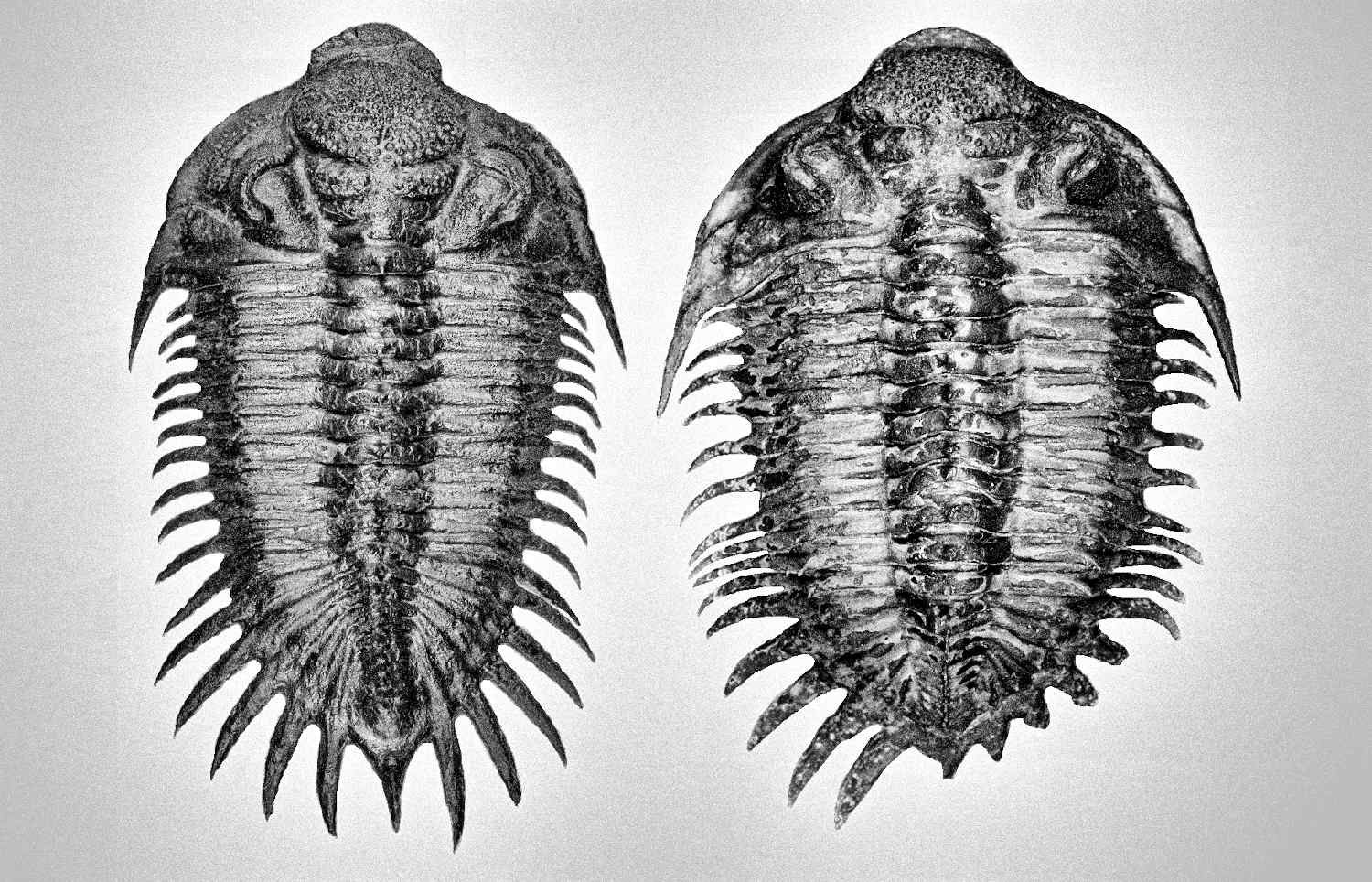


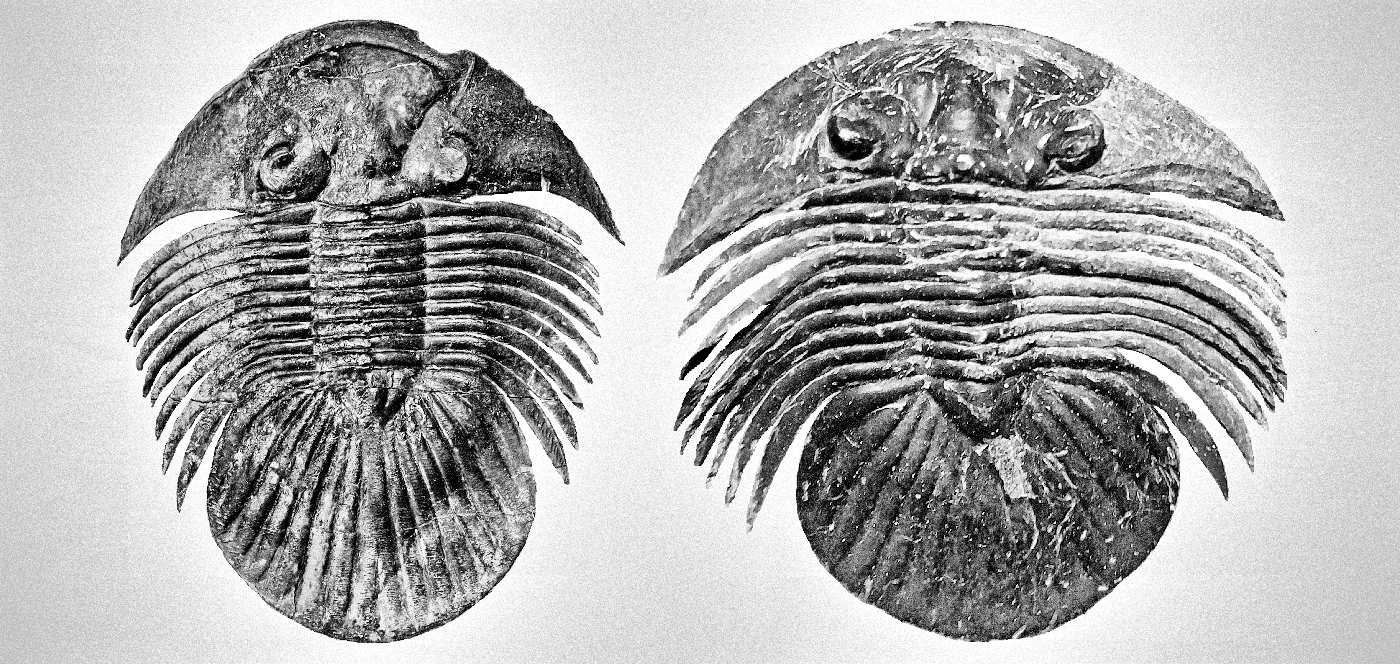







Generally, trilobites will show a more distinct shape between male and female under juvenile stage compares to adults (refer to Figure 7, 49 and 54). The body shape gradually becomes slightly indistinguishable when close to adult stage due to the increase in body size (male body become wider, refer Figure 51 compare to 52). But certain species will show more obviously in adult stage especially for species contain a unique super elongated male (refer to Figure 16 and 30).
Under some very rare condition, some trilobites might show a more obvious distinct spine length between male and female, especially on its genal spine (a spine located on both side of its head/cephalon) like show in Figure 36. Zlichovaspis rugosa is one of the species found have more obvious different spine length between its male and female, which is largely contribute by its male appear more longer in body length compare to its female.
There are another form of sexual dimorphism that can be easily distinguished by its cephalon especially for species contain large or helmet shape cephalon like Cambropallas telesto show in Figure 44 and Harpetid trilobite show in Figure 45 & 45A. The male will often appear oval shape of cephalon while female appear more spherical in shape.





Sexual Polymorphism and Specific Structural Behavior of Trilobites
During analyzed large amount of trilobite’s samples, the female species is observed often preserved into abdomen bending posture (especially at the end of last few segments, like Figure 63, right specimen). The dead posture of this bending shape does not seem to be a result from abdomen muscle contraction when the marine arthropods died for a period (as a result of each contraction muscle returns to their original position, which should be in “C” shape) as can be seen in dead shrimp posture. In fact, this is a typical posture of pregnant female shrimp (refer Figure 62). This observation is important to use as an additional identified method to differentiate a more complicated small variant morphology types of male and female trilobites.




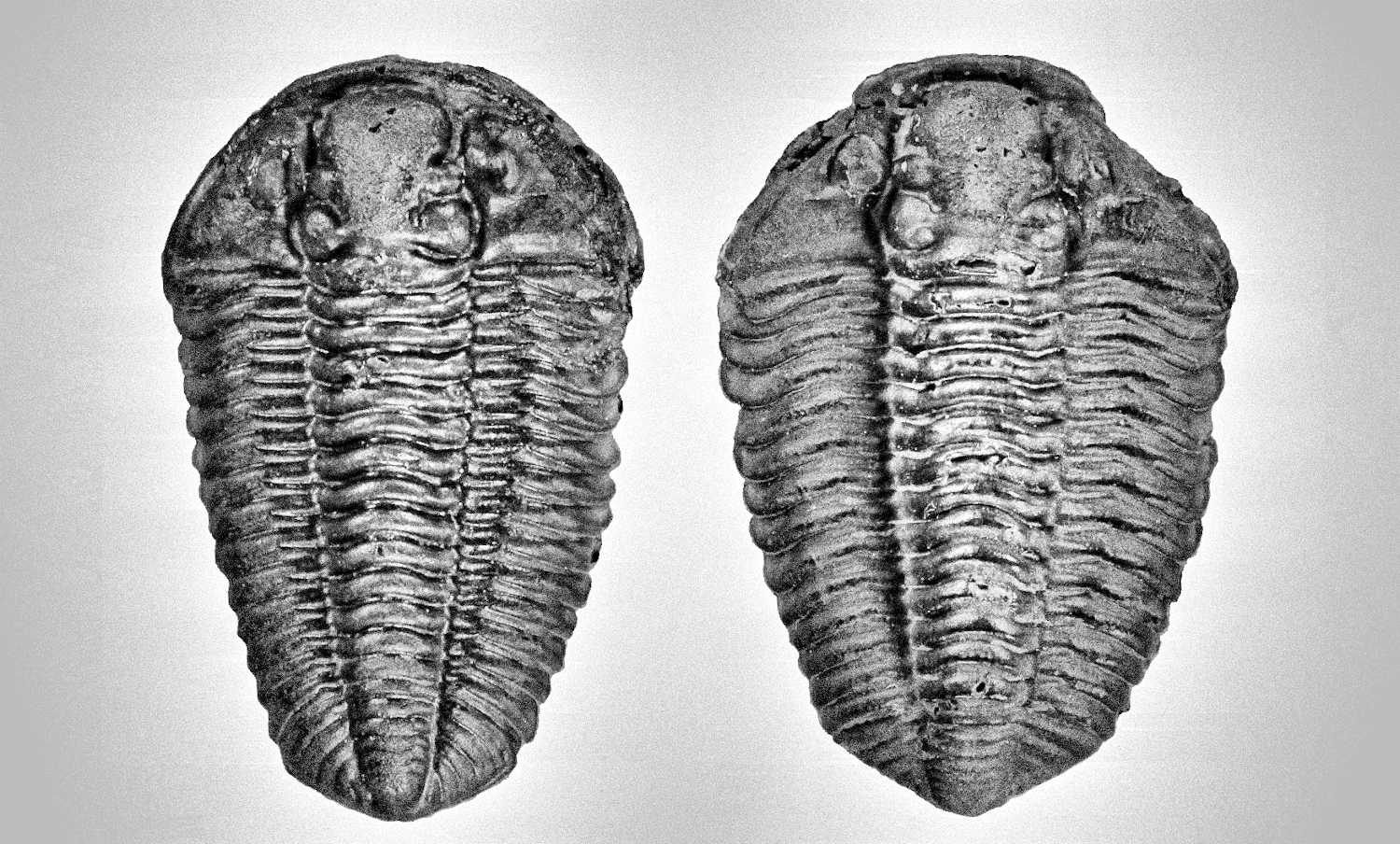
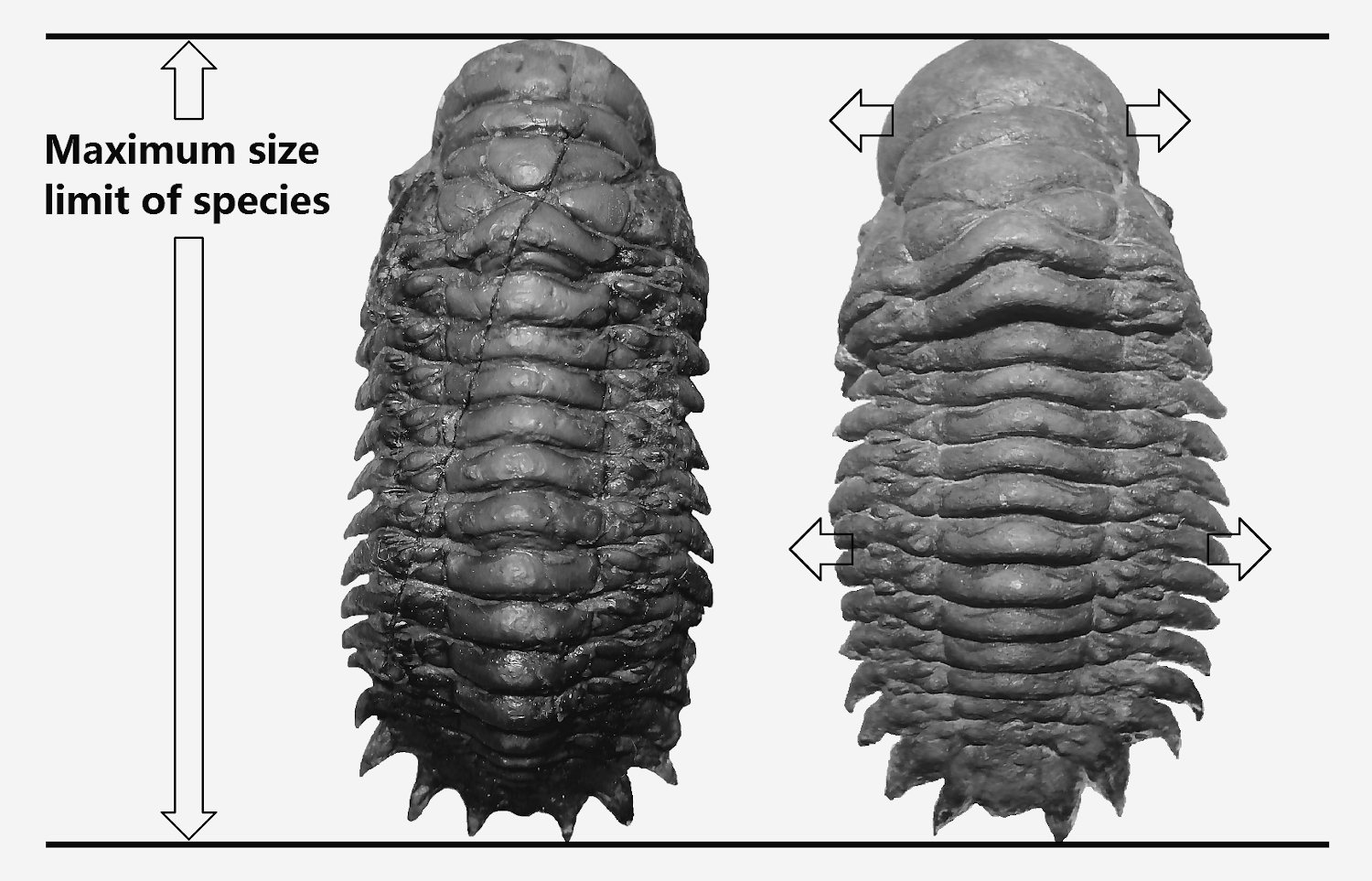
They are another form of trilobite shape that additional made complicated on distinguish the basal form of male and female morphology, apart from identify the rectangular shape (reason: rectangular shape make the identify point at end of the last few thorax segments of male similar to female) of small variance species like Morocconites malladoides and Gerastos (refer Figure 65). This form is spherical and wider transformation of female into mature form. The female for most of the trilobites species will tend to growth into this mature form when its body growth close to its maximum size limit. Under the same species, each individual will have its own maximum size limit, for example of Crotalocephalus gibbus (Figure 64), the typical maximum size limit is about 8 to 9 cm, influence by its own individual genetic factor, growth or sex hormone level, and environmental factor (food source), some individual will show early reach its maximum size limit (for current example is about 7cm on right specimen of Figure 64) and start transform into mature form, the whole body will expand to both side instead of further increase on its length, which form an additional spherical shape of similar species under the similar size when compare to other same breed. These types of mature form can still easily identify in Crotalocephalus gibbus but is difficult to identify in Gerastos. Figure 65 show a series of various growth stages of female Gerastos granulosus from B to D. The spherical or wider body shape of a full mature female will show a very distinct body shape compare to its youngest form especially for rectangular types of trilobites and often refer to geological compressed specimen or misclassified as new species. Figure 66 provide a different viewing angle to prove the specimen is fully inflated and does not suffer any geological deformed. There are still have another few species example of this fully mature form but lack of species name so will not discuss over here.


Apart from sexual dimorphism and mature form of morphology, under some condition, discrete variation within sexes is also occurs in trilobites. Some male will growth like a female like body, and some female will growth like a male. This phenomenon is known as sexual polymorphism. Primary suspect the hormone level different in some individual or food source in environment encourage the growth of these types of male and female polymorphs. To study and understand this mechanism, it required to find a good reference sample especially an easily breeding species and posses a strong sexual dimorphism. Guppy is one of the good example exhibit a strong sexual dimorphism (very distinct appearance between male and female, Figure 67) that suitable use to explain the polymorphism phenomenon for trilobites. Figure 68 show a special sexual polymorph of guppy. Under a same breed of offspring, they are often found few individual of female-like male morphs growths extremely faster and giant compare to the same breed. This types of male usually have a body very similar to female (a reason to be bigger) and low in color. This might indicate they might low in male sex hormone and slightly higher in female sex hormone that makes them growth bigger and faster. For male-like female morphs, they are often found in high colored species. These types of female individual is believed its body contains very high level of male sex hormone lead to male-like slim body and have very high in color. When understand the general polymorphism characteristic in guppy, it will help to explain this phenomenon in trilobites.


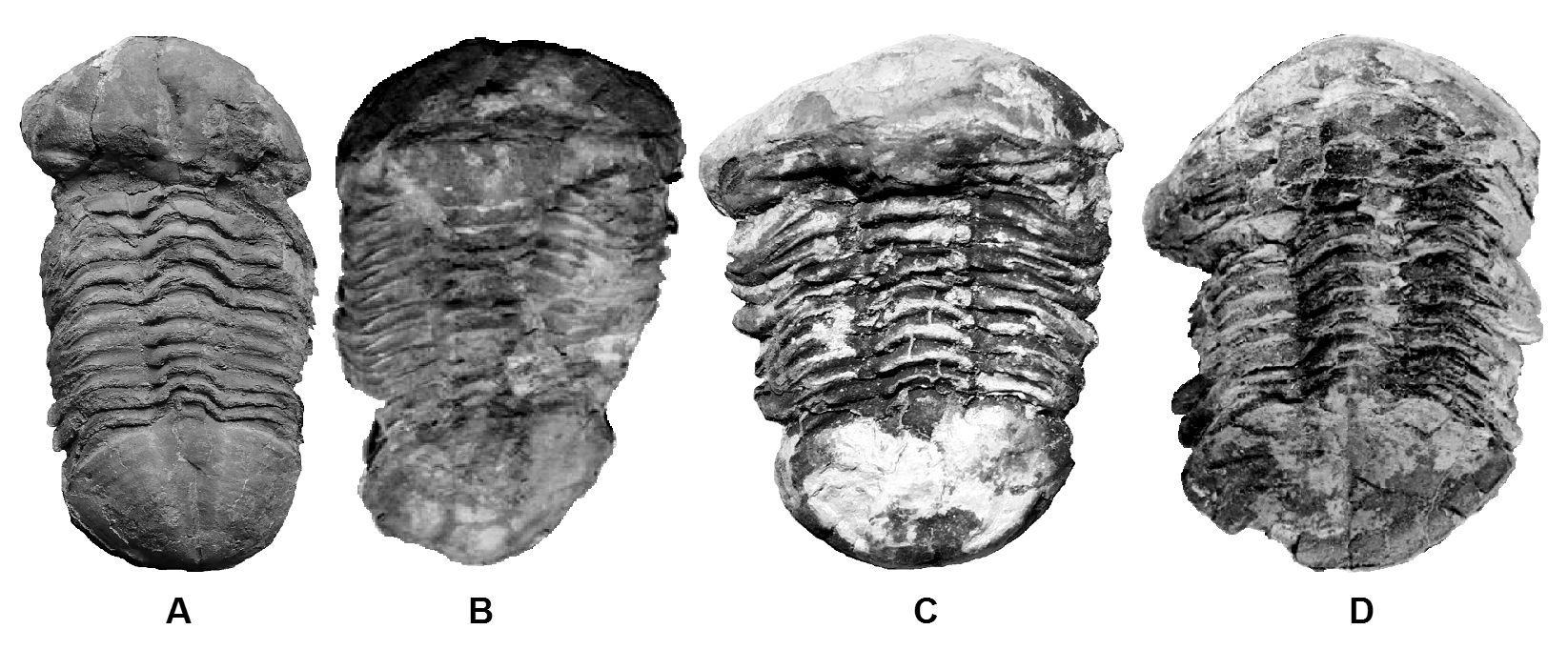
Figure 70 show a series of sexual polymorphs for trilobite species call Ductina vietnamica. Ductina exhibit a strong sexual dimorphism that is suitable used to study its polymorphs. Figure 70A & 70D belong to basal form of male and female. Figure 70B & 70C belong to sexual polymorphs. Although the current study is based on limited specimens of sexual polymorphs, but the female-like male morphs like Figure 70B usually will appear larger body size compare to its same breed offspring, it is observed typically larger than the basal male form, and is trust the female sex hormone induces the growth of larger and wider form of body. In contrast, the male-like female morphs (refer Figure 70C) often found in smaller size, and might early transform into mature female form (more wider and spherical body, the overall dorsal view will look like belong to specimen suffered a geological compression). This form of polymorphs believes influence much by its high level of male sex hormone and lead to reduction on its body size. Additional, Ductina is expected to live in high murky and high cloudiness (high total suspended solids, TSS) water environment, based on its development into blind form of morphology and the matrix preservation condition when the specimens of Ductina found, the male-like female morphs could belong to high color species although Ductina does not develop its eye for selective evolution like sexual selection in high color form but the color is actually primary influence by the male sex hormone.
References
- Biomobst. Identification guide. Horseshoe crab monitoring site. http://horseshoecrabs.myspecies.info/content/identification-guide (2016)
- 小智小姐. YouTube. https://www.youtube.com/watch?v=86qHoPbSbZI (2017)
- Commercial specimen. Identity protected due to current paleontology academic does not encourage in fossil trading. Only inverted and part of the image use instead for illustration and study purpose.
- Twin Cities Guppies. Pinterest.
- Joshguppyfish. Facebook (2019)

More issues available on home page